ATOMIC STRUCTURE
Atomic structure deals with structure and component of an atom. The first scientist discovered that matter is made up of small particles called atoms. The term atom means indivisible particles. But later, different scientists put forward atomic models. These atomic models account for atomic structure. There are several atomic models which include the following:-
- Dalton’s atomic theory.
- Thompson’s atomic theory.
- Bohr’s atomic model.
- Rutherford atomic model.
- Wave particles duality nature of matter.
- Heisenberg uncertainity principle.
-
DALTON’S ATOMIC THEORY
Dalton’s atomic theory includes the following main points:-
- Matter is made up of small indivisible particles called atoms.
- Atom is neither created nor destroyed.
- Atoms of the same elements are similar especially in mass.
- Atoms of different elements are different especially in mass.
- Atoms of different elements when combine they do so in small ratio whole numbers.
RECENT MODIFICATION OF DALTON’S ATOMIC THEORY
Dalton’s atomic theory was modified because all points were not valid. This resulted into discover of modern atomic theory. The following include point of modern atomic theory:-
Matter is made up of small indivisible particles called atoms was not valid due to the existence of three particles in atom. Matter is made up of a small divisible particle called atoms.
| Particle | Nature of change | symbol | mass | Position |
| Proton | +1 | 11P | 1.00 | Nucleus |
| Neutron | 0 | 01n | 1.00 | Nucleus |
| Electron | -1 | -10e | 1/1840 | Shell |
Atom is neither created nor destroyed was not valid due to the existence of radioactivity therefore Atom can be created or destroyed by either nuclear fission or fusion.
Atoms of the same elements are similar especially in mass was not valid due to the existence of isotopes. Atoms of the same elements have either same or different mass.
Atoms of elements are different especially in mass was not valid. Atoms of different elements have either same or different mass.
Atoms of different elements when combine they do so in small ratio of whole number was not valid because different elements combine by using variable ratio of whole number.
THOMPSON’s EXPERIMENT (DISCOVERY OF ELECTRONS)
Thompson’s conducted an experiment to investigate if air conducts electricity. The following circuit was used during the experiment.
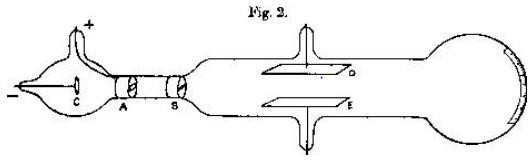
The emission tube have electrode at each end which is connected to the external circuit. The emission tube is connected to the vacuum pump in order to maintain the low pressure in the emission tube.
The circuit is switched on which results into the following observation:-
- The bulb emitted light which indicate that the gas conduct electricity.
- There is glowing of emission tube or emission of light.
- There is fluorescence of emission tube.
- The stream of rays running from cathode to the anode. Through investigation of properties of cathode rays by using magnetic field, electric field and gold electroscope results into
discover of electron. The cathode rays were the electrons.
EXPLANATION OF THOMPSON’S EXPERIMENT IN TERMS OF ATOMIC STRUCTURE
Ground state: Is lowest energetic state of an atom. Is a state when an electron filled in the lowest energy level before filling the highest energy level available. The electron filled in atom in order of increasing energy level. This state make atom to be stable.
Excited state: Is a state of an atom when electron filled in the highest energy level before filling lowest energy level available. If the electron excited jumps to the extent that the nuclear attractive force act upon it result pulling back of electrons. When return back to the ground state release all amount of energy which was absorbed inform of radiation.
RADIATION: This energy causes glowing of emission tube. When the radiation strikes the emission tube causes florescence of emission tube.
Convergent limit: Is a state of atom when an electron is removed completely from ground state to the infinite. The convergent limit occurs if atom gain high energy which result electrons to jump to the highest energy level where the nuclear attractive force cannot act up on it. This electron cannot return back to the ground state it result the atom left to positively charged. The convergent limit is a factor which causes some electrons to move from cathode to anode. These were stream of rays called cathode rays which later was electrons.
SIGNIFICANCE OF CONVERGENT LIMIT
These include the following:-
- It resulted into discover of the ionization energy. This ionization energy used in the inorganic section.
- It resulted into formation of ion particles. The ionic particle is more reactive when take part during chemical reaction.
- It resulted into production of rays. These rays are known as atomic spectrum.
THOMSON MODEL OF THE ATOM
After the discovery of electrons and protons, the next question was to know how these particles are arranged in an atom. The first simple model of the atom was proposed by J.J.Thomson in 1898.The Thomson atomic model is popularly known as the Thomson’s “plum-pudding” model of the atom.
Thomson considered an atom to be a sphere (radius = 10-10 m or 10-8 cm) of uniform positive charge into which the negatively charged electrons were embedded. This model is like plum- pudding dotted with raisins
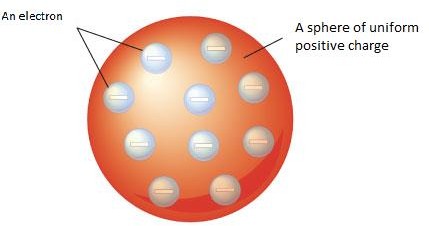
This model of an atom could not explain many experimental facts. So, it was abandoned.
RUTHERFORD’S SCATTERING EXPERIMENT
In 1911 Rutherford performed an experiment which is now known as Rutherford’s scattering experiment. In this experiment, he bombarded a thin sheet (0.00006 cm thickness) of gold with alpha α particles. The α-particles were obtained from a radioactive substance. The -particles are doubly ionized helium atoms (He2*).
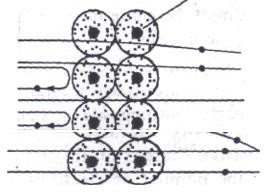
The scattered α -particles produced tiny flashes on striking with the zinc sulphide screen. These tiny flashes were observed with a movable microscope. The experimental set up used in the famous α-scattering experiment is shown in figure below. The following observations were made from the scattering experiment.
Structure of atom
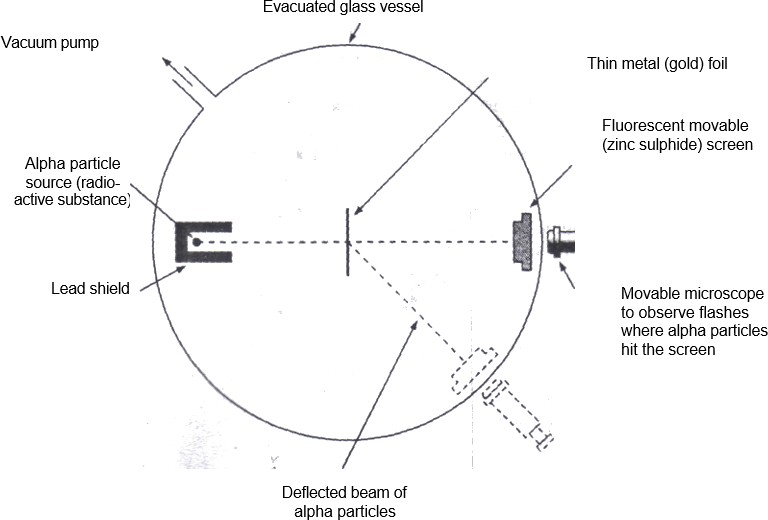
The α -scattering from metal foils. The -particles are produced by a radioactive source.Since lead absorbs α -particles a lead plate with a hole is used to obtain a beam of a particles.The α – particles scattered from the metal foil strike the fluorescent (zinc sulphide) screen and produce tiny flashes. A movable microscope is used to view the flashes.
- Most of the a-particles passed through the metal foil without any change in their path
i.e. they remained undeflected.
- Some of the α -particles deflected through small angles.
- Only a few of them (1 in 10,000) were actually deflected by as much as 90°, or even larger angles. One in 20,000 particles returned back suffering a deflection of 180°.
-
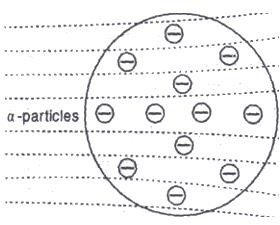
Explanation. The results of the scattering experiment could not be explained by the Thomson’s atomic. Calculations showed that a charge spread over a sphere of radius 10-8 cm could deflect α– particles only through small angles. The deflections of onlyα -particles through larger angels as observed would be possible If the positive charge in the atom is spread over a sphere of radius of about 10-13 cm thus, the α-particles scattering result could not be explained by Thomson’s atomic model.
RUTHERFORD’S NUCLEAR MODEL OF THE ATOM
On the basis of α-particle scattering experiment, Rutherford put forwarded in 1912, his nuclei model of the atom. According to this assumptions.
- An atom consists of a positively charged nucleus surrounded by a system of electronics.
Electrons are moving around the nucleus. The positive charge of the nucleus is due to the protons present in it.
- Electrons and the nucleus are held together by coulombic force of attraction,
- The effective volume of the nucleus is extremely small as compared to the effective volume of the atom. From the experiments, it was found that, Approximate radius of the
nucleus of an atom 10-14– 10-15 m (10-12 – 10-13 cm), Approximate radius of sphere of electrons (or the radius of an atom) 10-10 m (10-8 cm).Since, the volume varies as r3,
hence the volume occupied by the nucleus is about 1012 times the volume of the atom.
- Almost the entire mass of the atom is concentrated in the nucleus.
- The positive charge on the nuclei of different elements are always integral multiples of the electron charge, but opposite in sign. Since, each atom is electrically neutral, hence in
an atom, the number of positive charges on the nucleus of an atom is equal to the number of electrons in it. The Rutherford’s model of an atom is shown in the figure below
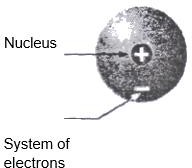
Rutherford, in 1912, put forwarded an atomic model. According to this model an atom consists of positively charged nucleus which is surrounded by a system of electrons. The electrons and I nucleus are held together by electrostatic forces. Because of the electrostatic attraction between the nucleus and the electrons, the electrons should ultimately fall into the nucleus. But, it does not happen. In order to explain why electrons not fall into the nucleus, Rutherford postulated that the electrons are not stationary, but are revolving about the nucleus in orbits. But, this explanation did not solve the problem completely. The physicists had observed that a revolving electric charge must emit radiation and thus lose energy. Thus, an electron revolving around a nucleus in an orbit should emit radiation and lose energy
If so, the continuous loss of energy should slow down electrons. As a result, the electron will not be able to stand the attraction of the nucleus and gradually move towards the nucleus. The electron should, therefore, follow a spiral path and ultimately fall into the nucleus within 10-8s. The atom should thus, collapse. But, it does not happen, as the atoms are stable. Thus, there must be something wrong in the Rutherford’s nuclear model of the atom.
RUTHERFORD ATOMIC MODEL
- The atom consists of extreme denser region at center called nucleus which have positive charged particles.
- The negative particles (electron(s) of atoms revolve around the nucleus in a path called orbit.
- The large area of atom is empty space.
SHORT COMING OF RUTHERFORD ATOMIC MODEL.
Rutherford atomic model accounted that electron revolve around the nucleus in the path called orbit. This is an imagination because no any scientist proved occurrence but discovered that when charged particle revolve around the opposite charge reach a point when they collapse due to the attraction force occurs between the nuclear and electron(s) hence electron capture. But this proved the truth of Rutherford because no any atom discovered to collapse.
BOHR’S ATOMIC MODEL The electrons revolve around the nucleus in a path called orbits which have certain energy.
The electron when revolve in stationery state does not radiate energy.
The electrons emit or absorb energy when shift from one energy level to another.
The electronic motions are those which its angular momentum is integral multiple of where n = 1, 2, 3…
SHORT COMING OF BOHR’S ATOMIC MODEL
Bohr’s accounted that electrons revolve in a single plane. But discovered that movement of electrons is not restricted in only on plane.
The Bohr’s model did not explain spectral of multi-electrons atom but accounted only spectral line of uni-electron hydrogen atom.
The Bohr’s model did not account for electrons which were found in a chemical bond.
Bohr viewed an electron as being placed at a certain distance from the nucleus. However, it was proved by Weaver Heisenberg’s Uncertainity Principle:–It is impossible at any
movement to predict the exact position and velocity of an electron in an atom.
MASS SPECTROMETER
Is an instrument which is used to determine the relative atomic mass of an element.
Mass spectrometers have an ability to determine the relative molecular mass of a compound and formula of a compound. In a spectrographic plate.
Mass spectrograph is a plate of mass spectrometers which detect and record the relative atomic mass of an element. The spectrographic plate is a detector or recorder. The following is a diagram of mass spectrometer which has different part.
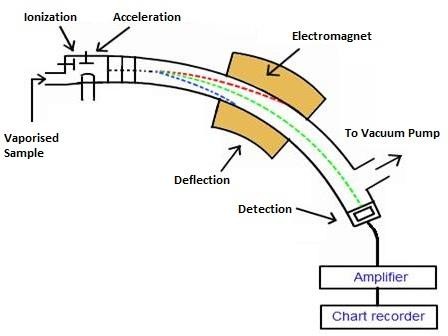
Vaporization. The instrument is evacuated in order to prevent the interference of air. The solid sample must be heated until it forms gaseous atoms and vaporized before it’s introduced into the mass spectrometer.
Ionization. The vaporized sample of element is introduced in the ionization chamber. This region has electric filament which emits fast moving electrons. These electrons collide with atom of an element as a result the atom split which leave only nucleus of positive charge. The nucleus left is where mass of atom is concentrated.
Acceleration. The ion particles formed in the ionization chamber are accelerated by two plate of negative charge toward the magnetic field. These two plates are connected to negative potential which accelerate ions as a beam of light toward the magnetic field.
Deflection. The beam of ions deflected in the magnetic field. The extent of deflection depends on the mass charge ratio m/e. The light particle deflected more than the heavy particles. The deflected ions strike on the detector. The ions at the same mass charge ratio strike on the same mass spot.
Detection. Once the ions strike the detector in the collector that converts the intensity of the ions into electric signals which is amplified by the amplifier into large electric current.
Recording. The current is used to operate a pen that moves on a paper tracing the peak of the isotope. The ion is recorded in terms of atomic mass and relative abundance. When they
fall on the photographic plate they produce a mass spectrum consisting of a series of line at different point. The mass of the ions detected on the photographic plate. Relative
abundance is the percentage of an isotopic atom in the element. The ions particles when strike the detector produce mass spectrum. The mass spectrum recorded as a peak.
The following include example of mass spectrum.
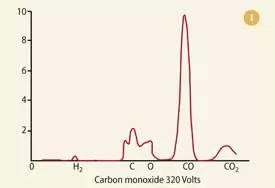
CALCULATION OF RELATIVE ATOMIC MASS
Relative atomic mass of an element is the average value of all the known isotope atomic weight relative to the proportional abundances. Relative atomic mass is more useful in chemistry than its simple atomic mass of element because simple atomic weight does not consider existence of isotopic forms of elements. The relative abundance of isotopic element is applied to determine relative atomic mass. Example isotopic element X contained Y% of zaX and W% of zbX, the relative atomic mass of element X is determined as given below.
![]()
Since y + w = 100
The length of peaks of each isotopic atom applied to find the relative atomic mass. Each atom have peak due to the different mass charge ratio m/e.
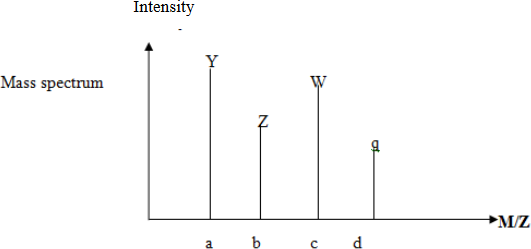
![]()
ATOMIC SPECTRUM
Definition: Are light waves which have definite line and colour because they have intermediate wave length which cannot be detected by a human eye. These spectrums have no harmful affect on human. Atomic spectrum produced once the atom gain energy which cause the electron to be excited and jump from lowest energy level. This results into an atom being unstable in order to maintain the stability these electrons return back to its group state which accompanied with release of energy inform of radiation. These radiation have wavelength which detected by a human eye of definite colour definite wave and definite line.
Continuous Spectrum: are spectrums which contain all possible frequency over wide range of energy. Continuous spectrums are colourless and have no definite line because they contain very short wave lengths which are not detected by an eye. The continuous spectrum recorded in a spectrographic plate as given below.
 Line spectrum: is a spectrum which consists of scattered definite line. These spectrums have very long wave length. The spectrographic plate of line spectrum
Line spectrum: is a spectrum which consists of scattered definite line. These spectrums have very long wave length. The spectrographic plate of line spectrum
Band Spectrum: Band spectrum are spectrums which consists of a group of definite line in small bands. The spectrographic plate of band spectrum includes the following:
BOHR-ATOMIC THEORY
(i) H – SPECTRUM Definition:
Is a definite line and colour which resulted when electric discharge is passed through hydrogen gas in the emission tube under very low pressure. The H – spectrum recorded in the spectrographic plate. The following is a horizontal diagram of H-spectrum
| UV | Violet | Infrared
Red |
Red | |||||
| X – ray | Radio electron | |||||||
| – ray | Television
wave |
|||||||
| UV – ray | ||||||||
| Invisible | ||||||||
Note: wave length increases
The explanation of horizontal diagram in terms of atomic structure:-
First band: is a colourless band or invisible band. These are spectrum produced by electrons which excited from the first shell. The electron from the first shell experience stronger nuclear attractive force which use high energy in order to jump toward highest energy level. When these electron returned back release high amount of energy which have shorter wave length. These wave lengths are not detected by a human eye. The radiations formed are continuous spectrum such as X – ray, sun rays etc.
Second band: is a visible band which has definite line and colour. These are line spectrum, produced by electron which excited from the second shell. The electron in the second shell need moderate energy in order to jump towards highest energy level when returned back release a normal energy which its wave length detected by a human eye.
Third band: Is invisible band. These spectrums are colourless and have no definite line.The electrons which produce these spectrum caused the scattered spectrum which appear as a colourless band. This is due to the lowest energy and highest wave length. The following include vertical diagram of H-spectrum.
The vertical diagram of Hydrogen Spectrum
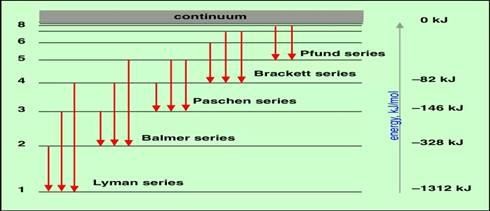
Lyman series: is a series of spectrum which resulted by electron excited from the first shell (n
=1). These series correspond to the invisible band or colourless band.
Balmer series: is a series of spectrum which resulted by the electron which excited from the second shell (n = 2) this corresponds with visible or violet band.
Paschen’s series: is a series of spectrum which resulted with electron excited from the third shell (n = 3) etc.
Bracket series: is a series of spectrum which resulted with electron which excited from the forth shell (n = 4) etc.
p-fund series: is a series of spectrum which resulted with the electron which exited from the fifth shell (n=5).
PLANCK’S QUANTUM THEORY
Planck put forward the Planck’s quantum theory. This theory has three main points which include the following;-
- Any radiation should be association with energy.
- The energy is released inform of radiation, occur in small packets called quanta. iii.The energy is directly proportion to the frequency.
![]() E f
E f
E = hf… (1)
Since h = Planck’s constant
h = 6.63 x 10 -34 JS
n= 1
![]() Since f =
Since f =
![]() E = (2)
E = (2)
C = 3.0 x 108 ms-1
REYDBERG EQUATION
Reydberg put forward a principle which applied to find wave length of spectrum. The wave length of H-spectrum determined is applied to find frequency and energy of the H- spectrum.
![]() The wave number is inversely proportional to the square of energy level differences ( n2).
The wave number is inversely proportional to the square of energy level differences ( n2).
V
![]()
![]()
![]() 1/V = RH n2
1/V = RH n2
![]() V = RH
V = RH
![]()
![]()
![]() Since V = 1/λ
Since V = 1/λ
![]() = ………………. (3)
= ………………. (3)
Where λ= wave length n1 = lowest energy level
n2 = highest energy level
RH = Reydberg constant RH is 1.09 x 107m-1
The value of n1 and n2 for H-spectrum can be obtained if given number of line and number of series. The value n1 is equal number of series given. But the value n2 is equal to the number of line plus the number of series. Example the value of n1 and n2 of third line of Balmer’s series include the following:-
Number of line = Third line Number of series = Balmer’s series n1 = number of series = 2
n2 = number of line + Number of series
= 3 + 2
n = 5
![]() But:
But:
= 1.09 X 107m-1[1/22-1/52]
= 1.09 X 107m-1[1/4-1/25]
= 1.09 X 107m-1[(25-4)/100]
= 1.09 X 107m-1[(21/100]
![]() λ = 4.45 x 10-7m of third line
λ = 4.45 x 10-7m of third line
![]() E=
E=
E=6.3 x 10-3 Js x 3.0 x 108ms-1
![]() 4.45 x 10-7m E=4.25 x 10-19J Also;
4.45 x 10-7m E=4.25 x 10-19J Also;
f =
![]() f =
f =
f = 6.74 x 1014s-1 f = 6.74 x 1014Hz
TRANSITION ENERGY
Is the energy which is required to shift electron from one shell to another. The energy required to shift electrons from one shell to another should be equal to the energy difference between those two shells. The energy difference between lowest energy level E1 and highest energy level E2 is obtained by using the following expression;-
E = E2 – E1… (1)
The trend of transition of electrons include the following; E = E2 – E1 – Transition take place from E1 – E2 exactly.
E > E2 – E1 – Transition take place from E1 toward above E2
E < E2 – E1 – Transition take place from E1 and hang between E1 and E2
But if electrons gain enough energy which is equal to the ionization energy result the electron to jump completely from ground state to infinite. These electrons cannot return back instead leave atom ionized positively. The energy supplied to the atom results to be excited and electron move completely from the ground state to the infinite. The energy supplied to the electrons is used as ionization energy and as kinetic energy. The ionization energy of electrons is equal to the amount of energy associated by electron in the shell or quantum number where it belongs.
The following expression is used to find the energy associated by electrons or energy of that shell.
1/λ = RH
![]() Let n1 = n n2 = ∞
Let n1 = n n2 = ∞
![]()
![]() since 1/ = 0
since 1/ = 0
![]() 1/λ = (i)
1/λ = (i)
E = hc/λ E/hc = 1/λ (ii)
Substitute (ii) into (i)
![]()
![]()
![]()
![]() = E = E =
= E = E =
Since R, h and C are constant 1eV = 1.6 x 10-19J
1MeV = 106eV
1MeV = 1.6 x 10-13J
But discovered that the energy increase from the nucleus atom toward the high energy level. The energy increases from first shell toward seventh shell. From seventh shell the energy is zero or the energy at infinite is zero. Then the energy from infinite which is zero towards the lowest shell decreases and results to be negative value of energy
| n∞ | = | 0.00ev |
| n7 | = | -0.15ev |
| n6 | = | -0.21ev |
| n5 | = | -0.54ev |
| n4 | = | -0.84ev |
| n3 | = | -1.50ev |
| n2 | = | -3.40ev |
| n1 | = | -13.60ev |
E = E2 – E1
= E – En
= 0 – En
![]() = 0 –
= 0 –
![]() E =
E =
The negative value occurs because the energy of infinite is zero. Since energy increase from nuclear towards highest energy level result the energy of each shell be negative value. This formula is used to determine energy of electron at each shell.
Example
- Find the first ionization energy of potassium.
- Find the wavelength, frequency and energy of third line of Balmer’s series ( RH = 1.09 x 107m-1) iii.If the wavelength of the first number of Balmer’s series is 6563Å. Calculate Reydberg constant and
wavelength of the first member of the Lyman series. iv.Find energy associated with electrons in the quantum number 2.
Solution:
-
- E = I.E
![]()
![]() k = 2:8:8:1 E =
k = 2:8:8:1 E =
E =
= -1.36 x 10-19J
E = E2 – E1
![]() E = 0 – -1.36 x 10-19 J E = 1.39 x 10-19J
E = 0 – -1.36 x 10-19 J E = 1.39 x 10-19J
-
- n1= 2
n2 = 3 + 1
![]()
![]()
![]()
![]()
![]()
![]() λ =
λ =
![]() λ= 4.86 x 10-7m
λ= 4.86 x 10-7m
-

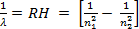 λ = 6563A From
λ = 6563A From
![]()
![]()
![]()
![]()
![]() RH =
RH =
RH = 1.09 x 10-3 A-1
RH = 1.9 x 10 -3 x 1010m-1 RH = 1.09 x 107m-1
-
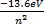 E =? n = 2
E =? n = 2
E =
![]() E = J
E = J
E= -5.44 x 10-19J
Example
![]()
![]() The U.V light has a wave length 2950 Å. Calculate its frequency energy 1A = 10-10M Given J
The U.V light has a wave length 2950 Å. Calculate its frequency energy 1A = 10-10M Given J
![]() J
J
If the electron dropped from E4 to E2: Find the frequency and wave length energy release Given line spectrum
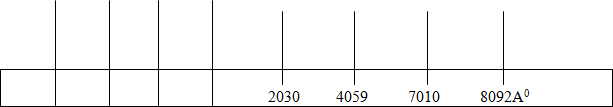
Wave length
- Which line has highest frequency and energy?
- Which line has lowest frequency and energy?
- Find the energy and frequency of each line in (i) and (ii) above?
| E5 | |
| E4 | |
| E3 | |
| e0 | E2 |
| E1 |
State either or not the transition of electrons would occur if energy supplied is
- Greater than E4 – E2
- Equal to E4 – E2
- Less than E4 – E2
- Greater than E3 – E2 but less than E4 – E2
- Smaller than E4 – E2

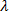 Solution:
Solution:
a) = 2950A
f = c
λ
![]() f =
f =
f = 1.02×10-2 S-1
E= hf
E = 6.3 x 10-34Js x 1.02 x 10-2 S-1 E = 6.426 x 10-36J
b) E4 = -1.36 x 10-19J E2 = -15.44 x 10-19J E = E2 – E1
E = E4 – E2
E = (-1.36 x 10-19) – (-5.44 x 10-19)
E = 4.08 x 10-1
But:
![]()
![]() E = hf f =
E = hf f =
f =
f = 6.15 x 1014 S-1
![]()
![]() Hence: F =
Hence: F =
λ =
![]() λ =
λ =
λ = 4.88 x 10-7m
![]() C. i) A line of wave length 2030A
C. i) A line of wave length 2030A
-
- A line of wave length 8092 A
 f =? E =?
f =? E =?
Line of 2030A
![]() Since f =
Since f =
![]() f =
f =
f = 1.48 x 1015S-1
E = hf
E = 6.3 x 10-34Js x 1.48 x 1015
![]() E=9.32 X 10-19 J
E=9.32 X 10-19 J
- Solution
- Greater than E4 – E2 result transition of electron from E1 and above E4
- Equal E4 – E2 transition take place from E2 to E4
- Smaller than E4 – E2 result transition of electrons from E2 but cannot reach instead hang between E2 and E4
- Greater than E3 – E2 but smaller than E4 – E2 result transition of electron from E2 and above E3 but cannot reach E4
THE QUANTUM THEORY
WAVE PARTICLE DUALITY NATURE OF MATTER
States that: “Matter has particle nature as well as wave nature”. This means that matter have dual properties or two properties such as particle nature and wave nature. The wave particle duality nature of matter was put forward by De Broglie scientist. De Broglie’s derived an expression which applied to find the De Broglie’s wave length.
De Broglie’s wave length is in terms of mass and momentum. Hence from Einstein equation;
E = mc2 (i)
![]() From Planck’s equation
From Planck’s equation
E = (ii)
![]() Compare equation (i) and (ii) mc2 =
Compare equation (i) and (ii) mc2 =
![]()
λ =
λ =
![]()
![]() De Broglie’s wave length in terms of energy From:
De Broglie’s wave length in terms of energy From:
λ = multiply by 1/c in both side
![]()
![]() λ = [1/c]
λ = [1/c]
![]()
![]()
![]()
![]() =
=
=
Since E = mc2
Example
Alpha particles emitted from radium have energy of 4.4MeV. What is the de-Broglie’s wave length?
The mass of moving particles is 9.01 x 10-19g. What is the de-Broglie’s wave length? The momentum of particles is 2.0 x 10-10gm/s. What is the de-Broglie’s wave length?
Given that:
h = 6.63 x 10-34 JS
C = 3.0 x 108m/s
Solution
- E = 4.4MeV
λ =?
![]()
![]() De Broglie’s
De Broglie’s
![]() =
=
λ =
λ = 4.52 x 10-13m
- Solution
m = 9.01 x 10-19g
λ =?
![]()
![]() De Broglie’s λ =
De Broglie’s λ =
λ =
![]() = 2.33 x 10-24m
= 2.33 x 10-24m
c. P = 2.0 x 10-10gms λ =? De Broglie’s
![]() λ =
λ =
![]() λ =
λ =
λ = 3.315 x 10-24Jg-1m-1s-1
HEISENBERG UNCERTAINITY PRINCIPLE
Heisenberg uncertainity principle state that, “It is impossible to determine position and momentum of electrons simultaneously with greater accuracy.” It is impossible to determined position and momentum of electrons because;-
- The size of electron is very small and as such radiations of high energy extremely small wavelength are required to detect it.
- Impact of these high energy photons changes both the direction and speed of the electron.
Thus; the very act of measurement disturbs the position of electron. The uncertainties in the determination of these two quantities vary inversely. If one is determined fairly accurately, the other must be corresponding less accurate. The distance of electron: Is a position of electron from the nucleus and momentum of electrons is product of mass of electrons and velocity of the electron, Heisenberg put forward an expression which is used
to determine uncertainity position and momentum of electrons.
Where: P – uncertainity momentum X – Uncertainity position
![]()
![]()
P
P = x
![]()
![]()
![]() P = (1)
P = (1)
![]() Since = proportionality constant
Since = proportionality constant
Also
![]() mc = (2)
mc = (2)
Example
The uncertainity in the momentum of particles is 3.3 x 10-16gms-1. Find accuracy with which its position can be determined
![]() X =
X =
![]() X =
X =
= 1.59 x 10-29m
WAVE MECHANICS
Wave mechanics deal with electron occupying space. The electrons revolve the nucleus through orbit and occur in the orbital.
Orbital:Is a region within an atomic sublevel that can be occupied by maximum two electrons that have opposite spin. Any orbital can accommodate a maximum of two electrons.
The energy level of atoms is a specific region around the nucleus electrons can occupy in atoms. A shell is defined as a complete group of orbital possessing the quantum number. The
electrons arranged in the atom starting from orbit from nucleus toward the orbit of highest energy level. The following include position of locating electrons in the atom.
- Principal quantum number (n)
The atom shells called principal quantum number (main energy level). The electrons filled in atom in order of increasing the energy from the nucleus. Each shell have specific constant number or electrons obtained taking
e = 2n2
| Old name | 1 | 2 | 3 | 4 |
| New name | K | L | M | N |
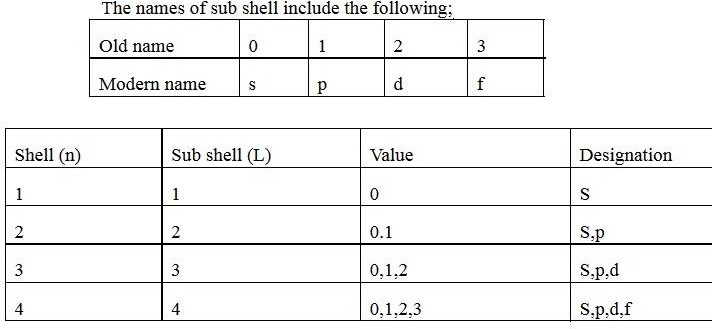
(ii)Subsidiary quantum number (L)
Subsidiary quantum number is a sub energy level. The sub energy level known as azimuthal quantum number, sub shell or sub energy level. The shell divides to form sub shell. The total number of sub shells in each shell is equal to the number of shell from the nucleus. This describes shapes of orbitals (sub-energy level).Values of subsidiary quantum number start from 0 n-1
The names of sub shell include the following;
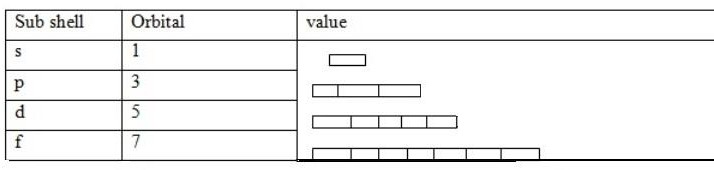
 iii. Magnetic quantum number (m)
iii. Magnetic quantum number (m)
This is a quantum number which describes the orbital of each sub shell. This sub shell divided and form orbital. Each sub shells have specific number of orbital.
 (iv) Spin Quantum number (s)
(iv) Spin Quantum number (s)
These quantum numbers describe the spinning of electrons in the orbital. Maximum number of electrons in the orbital in only two which spines in opposite direction. Since each orbital has two maximum electrons result the sub shell to have constant total number of electrons which accommodated in all shells S2, p6, d10, f14, g18, h22 and i26. In order to write electrons of element consider the knowledge of all four quantum numbers.
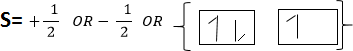
The following series of sub shell used to write the electrons structure.
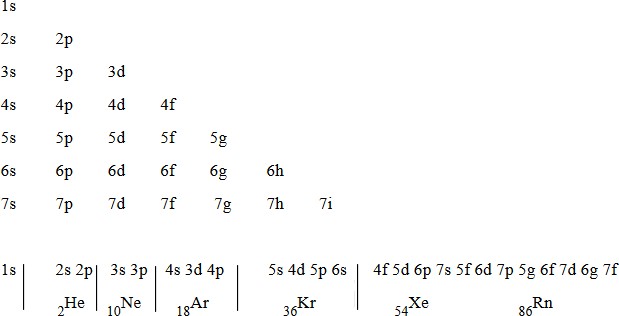
This arrangement used to write the electronic configuration.
Arrangement used to write the electronic configuration has some irregularities or abnormality. This irregularity occurs because there is overlapping of orbital. The orbital of low energy jump toward highest energy level and orbital of high energy drop down towards lowest energy level. Example 4s arranged first before 3d orbital.
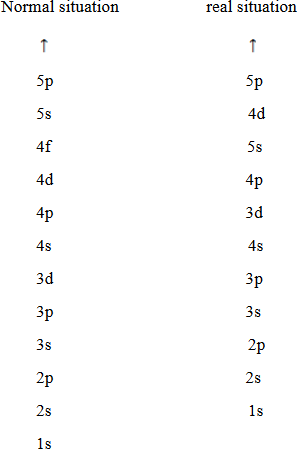
RULES WHICH GOVERN ARRANGEMENT OF ELECTRONS IN ORBITALS
- Aufbau’s principle – State that “in the ground state of orbital the lowest energy is filled by electrons before filling orbital of the highest energy”.
This means that electron filled in order of increasing energy level. The orbital of lowest energy when is full of electrons then the electron filled on the next orbital of highest energy level. Consider the following arrangement
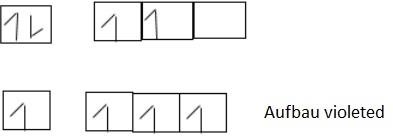
- Hund’s rule of maximum multiplicity
State that, “The electrons pair in the orbital of the same sub shell is allowed if all orbital of the same energy are occupied by electrons of maximum multiplicity. This means that the degenerate orbital are not allowed to pair up unless each orbital is singly occupied. The incoming pairing is done in an opposite spin. Consider a case with 5 electrons in valency shell.

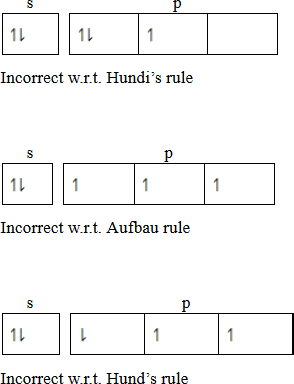
- Paul’s exclusive principle
States that, “Two electrons in an atom can never have exactly similar set of all quantum numbers.” This means that the maximum number of electrons which is occupied in the orbital is only two. Two electrons may occupy the same orbital only on condition that they have their spin in the opposite direction. Two electrons may have three quantum numbers the same; n, l and ml but the fourth, ms must be different.

Sometimes the electrons structure or electronic diagram of ions is written according to what kind of ions given.
There are two kinds of ions which include the following;-
Cation or metallic ion is formed through losing of electrons. The electrons which are lost occur in the orbital out the of the noble gas structure. The electron removed starting from orbital of low energy toward the orbital of highest energy.
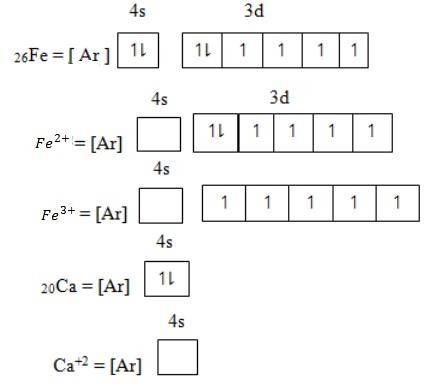
Anion or non-metallic ion – is formed by gaining electrons. Electrons which gain is added in the orbital having unpaired electrons starting from orbital of lowest energy to the orbital of highest energy level
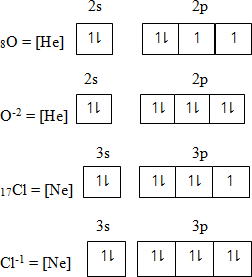
QUANTUM NUMBER
Is a number which describe the characteristics of electrons or is a number which is used to characterize electrons as they occupy orbitals in different energy levels. The quantum number is a number which describe main energy level, orientation of orbitals and spinning of electrons.
There are four type of quantum number which includes the following:-
- Principle quantum number (n): Is a number which specifies the location and the energy of electron. This is a number which describe the main energy level of electrons. The principle quantum number called shell orbit, main energy level and stationary state. The principle quantum number have specific name from the nucleus. K, L, M, N etc.
- Subsidiary principle quantum number (L): Is a number which specifies the shape of orbitals. Subsidiary principle quantum number is a sub energy level. The sub energy level is known as azimuthal number sub shell or sub energy level. The shell is divided to form sub shell. The total number of sub shells in each shell is equal to the number of shells from the nucleus. The names of sub shells include the following; s, p, d, f, g, h, i. The value of sub energy level i.e. 0, 1, 2, …L = n – 1.The value of L must be smaller that n because L = n – 1
| n | Value of L | Sub shell |
| 1 | 0 | 1s |
| 2 | 0, 1 | 2s2p |
| 3 | 0, 1, 2 | 3sp3d |
| 4 | 0, 1, 2, 3 | 4s4p4d4f |
| 5 | 0, 1, 2, 3, 4 | 5s5p5d5f5f |
- Magnetic quantum number: Is a number which specifies the number of orbital present in a given value of subsidiary quantum number. This is a quantum number which describes the orientation of orbital. The magnetic quantum number is a number of orbital. When the sub shell divides form orbital, these orbitals are called Magnetic quantum number.
1s – Orbital S = X
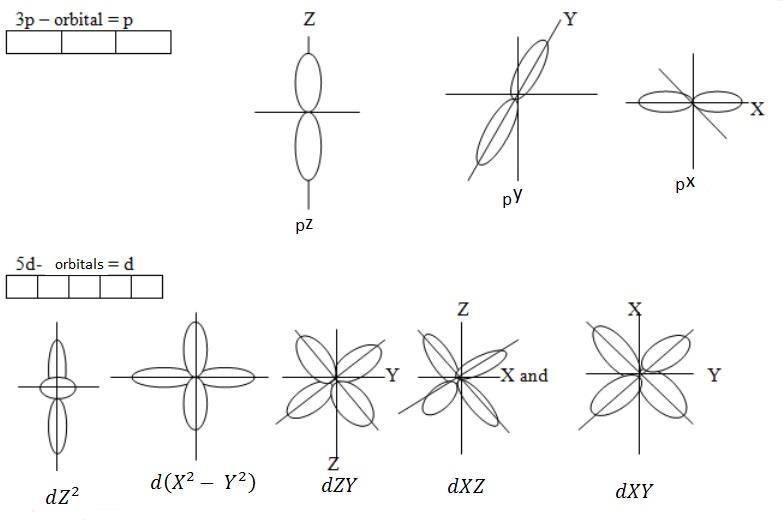
- Spin quantum number (s) – This is a quantum number which describes the direction in which the electrons spin. There are two maximum electrons, these electrons are moving in opposite direction. They revolve clockwise + ½ and another revolve anti – clock wise – ½
APPLICATION OF QUANTUM NUMBER
Quantum number is applied to find the number of sub shell (L), number of orbital (m) and total number of electrons (s) of the given quantum number (n). There are two methods which are applied to find total sub shells, orbital and electrons.
Find total number of electrons in the principle quantum number 2. (By using tree diagram)
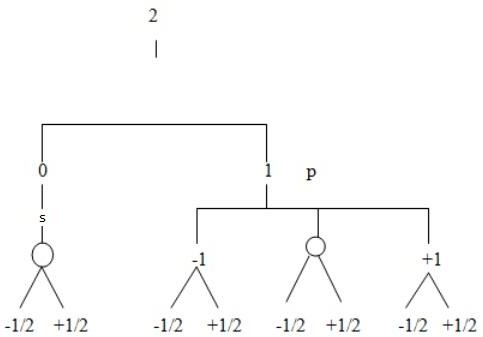
Total shells = 2 (s, p) Orbitals = 4 (0, 0, +1, -1)
Electrons = 8
Also: by using tabular form
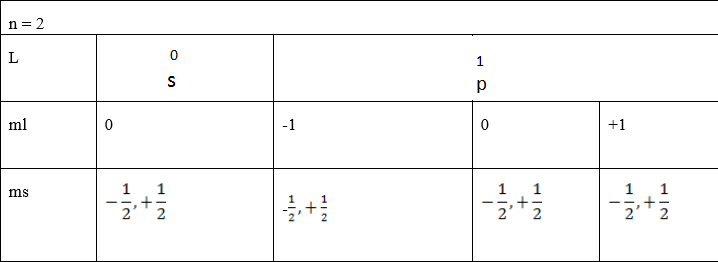
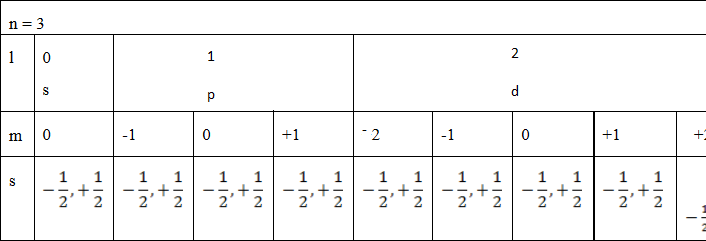 (b) Find the total number of electrons in the principle quantum number 3 Soln: (by using tree diagram)
(b) Find the total number of electrons in the principle quantum number 3 Soln: (by using tree diagram)
Example
- The modern theory of electron behavior is based on two assumptions. State them
- Define the following terms
- Orbital.
- Energy levels of atoms.
iii A shell.
iv. Quantum.
Solution:
- The modern theory of electron behavior is based on two assumptions.
- An electron has a dual nature i.e. it behaves both as a particle and wave.
- It is practically impossible to determine simultaneously both the position and momentum of an electron with any degree of precision.
- (i) An Orbital is a region within an atomic sublevel that can be occupied by a maximum of two electrons that have opposite spin. There are s,p,d, and f orbitals is a volume of space surrounding the nucleus within which there is a more than 95% chances of finding electrons. Any orbital can accommodate a maximum of two electrons.
- Energy level of atoms in specific region around the nucleus that electrons can occupy in atoms.
- A shell is defined as a complete group of orbital possessing the same quantum number.
- Quantum is the smallest countable, discrete packet or increment of radiant energy that can be absorbed or emitted.
Example
Explain the meaning of the following terms:
- Quantization of energy and radiation.
- Wave particles duality of matter.
- Quantum number.
Solution
- Light and other forms of electromagnetic radiations are not emitted continuously but in discrete “packets” called photons. Energy of an electromagnetic radiation is also not emitted continuously but in “packets” called quanta. A photon of radiation carries a quantum of energy. Energy and radiation are quantized in that electron shall fall from one energy level to another and not anywhere else in between.
- According to de Broglie, every sub – atomic particles has both wave and particle properties. Photons of light and other electromagnetic radiations, are regarded and wavelengths. They are regarded as waves because they have specific frequencies. Although electrons have extremely small mass, they have both velocity and momentum as particles.
- Quantum numbers are used to characterize electrons as they occupy orbitals in different energy levels. Each electron is characterized by four quantum numbers:-
The principal quantum number, n, which specifies the location and energy of the electron.
The azimuthal, subsidiary or angular momentum quantum number, l, which specifies the shapes of the orbitals.
The magnetic quantum number, m, which specifies the orbital orientation in space.
The spin quantum number, s, which specifies the direction in which the electron is spinning. These quantum numbers completely describe the stationary stage of the electron.
Example
- Distinguish between the following terms:-
- An orbital and orbit.
- S – Orbital and P – orbital.
- A quantum of light and quantization of energy.
- Quantum shell and quantum number.
- Explain briefly the meaning of the following quantum numbers:-
- n
- l
- m
- ms
- In a tabular form specify all the four quantum number for each electron in an atom whose n value is 2. Given all the orbitals are full of electrons.
- Given the value of the quantum numbers n, l and m for the electron with the highest energy in sodium atom in the ground state.
- Write down all the quantum number of all the electrons in the ground state of nitrogen atom.
- Give the values of all the four quantum number for 2p electrons in Nitrogen.
- Briefly explain why the following quantum numbers are not allowed. i) n = 1, l = 1, m = 0
ii) n = 1, l = 0, m = 2
iii) n = 4, l = 3, m = 4 iv) n = 0, l = -1, m = 1 v) n = 2, l = -1, m = 1
Solution
- (i) An orbit is a well define circular path in which electrons were assumed to revolve around the nucleus. Whereas an orbital is a three dimensional region of space around the nucleus whereby there is high probability of finding an electron.
- S–Orbital is spherical and hence non directional whereas p-orbital is dumbbell in shape and it is directional.
- A quantum of light is a photon of radiation emitted when an electron jumps from one energy level to another whereas quantization of energy means energy emitted or absorbed following electron transition between one energy level and another is not continuous rather it is in form of small packet called quanta.
- A quantum shell is the energy level of an electron in a given atom whereas quantum number refers to specifies way of defining an electron in a given atom whereas quantum number refers to specified way of defining an electron in a given orbital of an atom using n, l, m and ms values.
-
-
- (i) n – represents the principle quantum number;
-
It specifies the location and the energy of an electron. It is a measure of the volume of the electrons cloud.
As the value of n increases or becomes less negative it means the energy levels of the electrons get further away from the nucleus n can have only integers 1, 2, 3, 4 to infinite represented by K,L,M,N etc. Also called azimuthal quantum number or subsidiary quantum number, specifies the shapes of the orbital, when n = 1 there’s only l s orbital where n = 2, there’s 2s and 2p orbital, thus for a given value of n, l, are all from 0, 1, 2…up to l = n – 1. Thus when n = 4 values are 0,
![]() 1, 2 and 3. Which represents 4s, 4p, 4d and 4, â„“ sub–levels m Magnetic quantum number specifies the number of orbital present in a given value of â„“.
1, 2 and 3. Which represents 4s, 4p, 4d and 4, â„“ sub–levels m Magnetic quantum number specifies the number of orbital present in a given value of â„“.
It specifies the orientating i.e. the direction of the orbital to magnetic field in which it’s placed.
It accounts for the splitting of the spectral lines observed when an atom emitting radiation is placed in a magnetic field, ms – Refers to spin quantum number.
- It indicates the direction in which the electron spins.
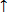
 Only two values are allowed for an electrons i.e. electrons may spin clockwise, shown as +1/2 or may spin anticlockwise as shown as ½ or
Only two values are allowed for an electrons i.e. electrons may spin clockwise, shown as +1/2 or may spin anticlockwise as shown as ½ or
| Principle (n) | Azimuthal | Magnetic quantum | Spins ms |
| n = 1 | â„“ = 0 | m = 0 | +1/2 , –1/2 |
| n = 2 | â„“ = 0
â„“ = 1 |
m = 0
m = -1 |
+1/2 , –1/2
+1/2 , –1/2 |
| m = 0
m = 1 |
+1/2 , –1/2
+1/2 , –1/2 Total of 10 electrons |
- When n = 3 in Na atom that electron is found in s – orbital thus â„“ = 0 and also ml = 0
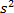 1 electrons:
1 electrons:
(1st) n = 1, l = 0, m = 0, s = +1/2 (2nd) n = 1, l = 0, m = 0, s = -1/2
Electrons:
1st n = 1, l = 0, m = 0, s = +1/2 (2nd) n = 1, l = 0, m = 0, s = -1/2
2 Px; n = 2, l = 1, m = +1, s = +1/2 2Py; n = 2, l = –1, m = –1, s =
+1/2
2Pz; n = 2, l = 1, m = 0, s = +1/2
-
-
- 2P electrons, there’s
-
2Px; n = 2, l = +1, m = +1, s = +1/2 2Py: n = 2 , l= –1, m = –1, s =
+1/2
2Pz: n = 2 l = 1 m = 0, s = +1/2
-
-
- (i) l value must be smaller than n value since l = n-1
- When l = 0 value of m is only 0
- For l= 3, m can range from –3 to +3. Thus m=+4 is not allowed
- n value cannot be equal to zero.
- l value cannot be a negative number.
-
Example
- briefly explain the meaning of the following quantum numbers:-
- ml
- n
- l
- ms
- If n = 2, tabulate the values of 1, ml and ms.
- Give the possible values of 1 and m, for an electron with the principal quantum number, n= 3
- Briefly explain why an electron cannot have the quantum number n = 2, l = 2 and ml = 2
Solution
(a) (i) ml – Magnetic quantum number with values from -l to +l. These are numbers showing the number of sub – level in each energy level.
– Describes orientation
- n – Principal (shell) Quantum number value of 1, 2, 3 etc theses are numbers used to represent the main energy level in which an electrons is found.
- l – Azimuthal or subsidiary or Angular or orbital Quantum number. They specify the shape of orbitals.
- ms – Magnetic spin Quantum number with values +1/2 and –1/2 shows the spinning of electrons in orbitals (clockwise and anticlockwise direction).
- For n = 2, the scheme below illustrates the value of 1, ml and ms.
l=0, 1
ml=-1, 0, +1, 0
ms= ±1/2, ±1/2, ±1/2, ±1/2
Therefore the total number of electrons = 8
- If n = 3, possible values of 1 and m are; l = 0, 1 and 2
m = 0 for l = 0
m = –1, 0 and +1 for l = 1
m = –2, –1, 0, +1 and 2 for l = 2 Note that: l=n-1
- An electrons cannot have quantum numbers, n = 2, l = 2 and m = 2. This is because when n = 2, l can be 0 and 1; then ml can be 0, –1, 0, +1 different values which can occupy in orbitals (i.e. they have n, l and m values the same but they must spin in opposite directions and hence have different ms values).
RULES FOR FILLING ELECTRONS IN THE ORBITALS OF AN ATOM
The orbitals of an atom can be filled with electrons by applying the following rules.
Aufbau Principle
This is also known as the building up principle. According to this principle “The electrons in an atom are so arranged that they occupy orbitals in the order of their increasing energy.”
Thus, the orbital with the lowest energy will be filled first, then the next higher in energy, and so on. Since, the energy of an orbital in the absence of any magnetic field depends upon the principal quantum number (n) and the azimuthal quantum number (l), hence the order of filling orbitals with electrons may be obtained from the following generalizations.
- The orbitals for which n + I is the lowest is filled first.
- When two orbitals have the same values of n+l, the orbital having the lower value of n is filled first.
The order of filling of various orbitals with electrons obtained by this rule is given below: 1s, 2s. 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f,5d, 6p, 7s, 5f…
To remember this sequence may be a difficult task. Given a long side is a simple way of working out this order. In this method a series of arrows running from upper right to the lower left gives the order of orbitals with increasing energy.
Pauli’s Exclusion principle
In 1925 Wolfgang Pauli discovered what is known as the exclusion principle. This principle is very useful in constructing the electronic configuration of atoms. According to this principle “No two electrons in an atom can have the same values for all the four quantum numbers”.
For example in 1s orbital of helium atom there are two electrons. According to the concept of quantum number and Pauli’s rule, their quantum numbers are:
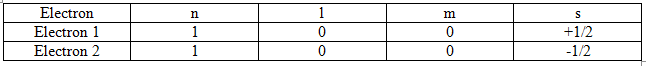
The + and – sign before j refers to the clockwise and anticlockwise spins of the electrons.
Thus, the two electrons having the same values of n, I and m could have different values
of s, i.e., their spins are in the opposite directions. This leads to a very significant observation that
“Each orbital can accommodate at the maximum two electrons with opposite spins.”
Applications of the Pauli’s exclusion principles
The Pauli’s exclusion principle leads to the following conclusions:
- An orbital cannot have more than two electrons.
- In any main energy level (shell), the maximum number of electrons is twice the in number of orbitals, or is equal to 2n2, where n is the principal quantum number.
Hund’s Rule of Maximum Multiplicity
The rules discussed above do not give any idea for filling electrons into the orbitals having equal energies (such states are called degenerate states). For example, three p-orbitals, i.e. px, py and pz, have equal energy. How should electrons be filled into these orbitals? Let us take an example in which three electrons are to be filled into three p-orbitals. The three electrons can be filled into three p orbitals in two different ways as shown below.
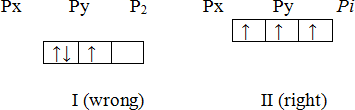
Now, which of the two is correct? The answer to this question is given by Hund’s rule, Hund’s rule states that,
“When more than one orbitals of equal energies are available, then the electron*,] first occupy these orbitals separately with parallel spins. The pairing of electrons will | only after all the orbitals of a given sub-level are singly occupied.”
According to the Hund’s rule, the correct way of filling three electrons in three p orbitals that in which each orbital is singly occupied, (arrangement II above).
Hund’s rule is also known as the Hund’s rule of maximum multiplicity.
Explanation
Two electrons with parallel spins, tend to be as far apart as possible to minimize the electrostatic repulsion. Therefore, the electrons prefer to occupy the orbitals singly
as far as possible. When all the orbitals get singly occupied, then the incoming electron has two choices either to pair up with the other electron or to go to the next higher orbital.
When vacant orbital of suitable energy is not available, then the incoming electron will have no choice except to pair up with another
electron.
Example
- State the;
-
- Heisenberg uncertainty principle.
- Hund’s rule.
- Paul’s Principle.
- Aufbau’s Principle.
-
- Brief explain why the following sets of quantum number are NOT allowed in hydrogen atom:
(i) n = 1, l = 1, ml = 0
(ii) n = 1, 1 = 0, ml = 2
- n = 4,1 = 3, ml = 4
- n = 0, 1 = 0, ml = 0 and (v) n = 2, 1 = –1, ml = 1
- How many orbitals are there in each of the following sublevel?
-
- 1s
- 2p
- 3d
- 4f
-
- How many sublevels are there in each of the following shells?
-
- K
- N
- L
-
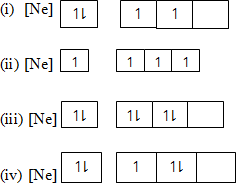
- Which of the following electronic configuration is correct for 14p in its ground state?
State the principle violated in each case
Solution
- (i) The Heisenberg’s uncertainly principle states that; “it is impossible to measure simultaneously the exact position and exact velocity (momentum) of an electron.” Any attempt to measure one quality will distract the measurements of the other quantity
- Hund’s rule states that, “electron pairing is not allowed until all orbitals of the particular energy sub – level are occupied by at least one electron.”
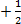
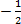 Paul’s Exclusion Principle, “states that no two electrons may have all the four quantum number the same.” Two electrons may have three quantum numbers of the same; the paired electrons are always in opposite direction at the same time.
Paul’s Exclusion Principle, “states that no two electrons may have all the four quantum number the same.” Two electrons may have three quantum numbers of the same; the paired electrons are always in opposite direction at the same time.
(E.g. and ).
- Aufbau Principle states that, “In electrons configuration, electrons are filled in order of increasing energy levels.” Thus the lowest energy available must be filled up first. The orbital energy increase in the following order; is 2s, 2p, 3s, 4p, 5p etc.
- (i) n = 1, 1 = 1, ml = 0 this is not allowed because l must be smaller than n, in the case l = 1 which is not allowed.
- n = 1, 1 = 0, ml = 2. This is not allowed because 1 = 0 and ml = 0
- n = 4, 1 = 3, ml = 2. This is not allowed because for 1 = 3 can range from –3, +3, thus, +4 is not allowed.
- n = 0, l = 0, ml = 0; this is not allowed because n cannot be zero.
- n = 2, l = -1, ml = 1. This is not allowed because l cannot be a negative number and n can never be = 0
- (i) 1 s sub energy level has one – orbital (which is 1 s – orbital).
- 2 p sub energy has three p – orbital (which is 1 s – orbital).
- 3 d sub energy level has five d – orbital [which are 3 d (x2 – y2), 3dz2 3dxy, 3 and
3dyz].
- 4 f sub energy level has seven f – orbital [which are 4xy, 4fyz, 4fz, 4f(x2 – y2), 4f(x2-z2) and 4f (y2 – z2)].
- (i) K has one sub level (which is l s2).
- N has four sub levels (which are 4s2, 4p6, 4d10 and 4f14).
- L has two sub levels (which are 2s2 and 2p6).
- Is the correct electronic configuration of 15p
This is correct since it obeys principle governing electron in an atom distribution
- [(Ne)]
- [(Ne)] aufbau (building up) principle is violated as it states that electrons available as the case here.
- [(Ne)]
- [(Ne)] Hund’s Rule of maximum that multiplicity is violated as it states electrons occupy orbital as singly as possible
Example
-
- State the rules used in filling electrons in various orbitals of an atom.
- By using 3 different ways for each of the following atoms write their electron configuration
- C
- N
- Ag
- Write the electronic configuration of Na+ and F– then show the other element whose electronic configuration resembles these ions. Show that electronic configurations of Cu and Cr are unusually written. Give reasons.
Solution
(a)(i)Paul’s exclusion principle states that; “no two electrons may have all the four quantum number the same i.e. electrons may occupy the same orbital only on conditions that they have their spins in the opposite direction.”
- Hund’s rule of maximum multiplicity states that, “when more than one orbital with equal energies are available, electrons tend occupy those orbital separately first with parallel spins and separately first with parallel spin and pairing of electrons will start only after all the orbitals of a given sub level are singly occupied.”
- Aufbau principle states that, “Electrons in an atom are so arranged that they occupy the orbitals in the order of their increasing energy.”
-
-
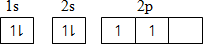 (i) C Atomic number 6 Using Box method:
(i) C Atomic number 6 Using Box method:
-
1s2 2s2 2p2
Using noble gas structure; [He] 2p2
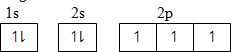 N atomic number 7 Using box method
N atomic number 7 Using box method
Using sub levels only;
1s2 2s2 2p3
Using noble gas structure;
[He] 2p3
- Ag atomic number 47
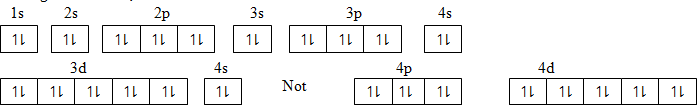 Using box method;
Using box method;
Note: 4d is filled first to maintain stability of full filled d – orbital before 5s is filled. Using sub levels only;
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 4d10 5s1
Using noble gas structure; [Kr] 4d10 5s1
(c)
- Na+ number of electrons also 10 1s2 2s2 2p6 resembles Ne
- F – number of electrons also 10 1s2 2s2 2p6 resembling Ne
(d) Cu Atomic number 29
e.g. [Ar] 4s1 3d10
Cr Atomic number 24
e.g. [Ar] 4s1 3d5 full filled and half filled orbitals are very stable electronic structures. The stability gained is the cause of 4s electrons to be unpaired and make 3d orbitals either full filled or half filled.
Note that: In writing the electronic configuration the atoms or orbitals of the same value are usually written together irrespective of their relation energy levels.
E.g. Cu 1s2 2s2 2p6 3s2 3p6 3d10 4s1 not 1s2 2s2 2p6 3s2 2p6 4s1 3d10. To save time and space noble gases are often utilized e.g. sodium [Ne] 3s1 and Iron [Ar] 3d6 4s2